L-岩藻糖检测试剂盒,K-FUCOSE

-
- 产地爱尔兰
- 品牌Megazyme
- 货号K-FUCOSE
- 规格100次
详细描述
L-岩藻糖检测试剂盒,K-FUCOSE 是一种简单,快速且可靠的方法,用于测量和分析植物提取物,生物样品和其他材料中的L-岩藻糖。该试剂盒可用于测量不作用于生色底物的α-岩藻糖苷酶。
内容说明: 如果将所有体积减半,则每个套件的手动测试次数可以加倍。使用MegaQuant TM 波长分光光度计(D-MQWAVE)可以很容易地适应这一点。
| 内容: | 100次检测(手动)/ 1000次检测(微孔板)/ 1020次检测(自动分析仪) |
| 运输温度: | 室温 |
| 贮存温度: | 短期稳定性:2-8 o C, 长期稳定性:参见各个组件标签 |
| 稳定性: | 在推荐的存储条件下> 2年 |
| 分析物: | L-岩藻糖 |
| 分析形式: | 分光光度计,微孔板,自动分析仪 |
| 检测方法: | 吸光度 |
| 波长(nm): | 340 |
| 信号响应: | 增加 |
| 线性范围: | 每次测定0.5至100 µg L-岩藻糖 |
| 检测限: | 0.68毫克/升 |
| 反应时间(分钟): | 〜10分钟 |
| 应用实例: | L-岩藻糖是岩藻依聚糖(一种海洋多糖),食品,药品和其他材料(例如生物样品等)中的主要成分。 |
| 方法识别: | 新颖的方法 |
-
非常划算
-
制备后所有试剂均稳定> 2年
-
仅提供酶试剂盒
-
简单格式
-
反应时间短(〜10分钟)
-
可从我们的网站上获得Mega-Calc ™软件工具,以进行无忧的原始数据处理
-
含标准
-
适用于手动,微孔板和自动分析仪格式
L-海藻糖检测试剂盒 L-Fucose Assay Kit 货号:K-FUCOSE Megazyme试剂盒
- L-海藻糖检测试剂盒
-
英文名:L-Fucose Assay Kit
-
货号:K-FUCOSE
-
规格:100 assays (manual) /
- Very cost effective
- All reagents stable for > 2 years after preparation
- Only enzymatic kit available
- Simple format
- Rapid reaction time (~ 10 min)
- Mega-Calc™ software tool is available from our website for hassle-free raw data processing
- Standard included
- Suitable for manual, microplate and auto-analyser formats
- Ensure that you have tested the standard sample that is supplied with the Megazyme test kit.
- Send the results of the kit standard, blank samples and the results obtained for your sample, in the relevant MegaCalc spreadsheet (if available) to Megazyme (cs@megazyme.com). Where available the relevant MegaCalc spreadsheet can be downloaded from where the product appears on the Megazyme website.
- State the kit lot number being used (this is found on the outside of the kit box).
- State which assay format was used (refer to the relevant page in the kit booklet if necessary).
- State exact details of any modifications to the standard procedure that is provided by Megazyme.
- State the sample type and describe the sample preparation steps if applicable.
- The easiest method is to use a microplate reader that has a path-length conversion capability (i.e. the microplater reader can detect the path-length of each well and convert the individual readings to a 1 cm path-length). This will allow values to be calculated using the MegaCalc calculation software which can be found where the product is located on the Megazyme website.
- Perform a standard curve of the analyte on each microplate that contains test samples and calculate the result of the test samples from the calibration curve (concentration of analyte versus absorbance).
- Perform a standard curve of the analyte in both the cuvette format (i.e. with a 1 cm path-length) and the 96-well microplate format and use these results to obtain a mean conversion factor between the cuvette values and the microplate values. Subsequent assays in the microplate format can then be converted from the calculated conversion factor.
市场价: 3392元
The L-Fucose test kit is a simple, rapid and reliable method, for the measurement and analysis of L-Fucose in plant extracts, biological samples and other materials. This kit can be used in the measurement of α-fucosidases that do not act on chromogenic substrates.
Suitable for manual, auto-analyser and microplate formats.
UV-method for the determination of L-Fucose in plant material,
polysaccharides, pharmaceuticals and other materials
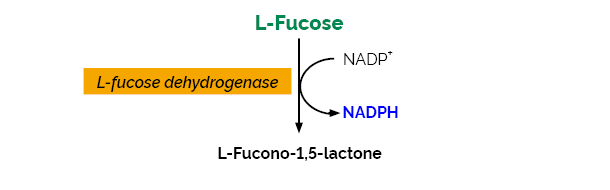
Principle:
(L-fucose dehydrogenase)
(1) L-Fucose + NADP+ → L-fucono-1,5-lactone + NADPH + H+
Kit size: 100 assays (manual) / 1000 (microplate)
/ 1020 (auto-analyser)
Method: Spectrophotometric at 340 nm
Reaction time: ~ 10 min
Detection limit: 15.4 mg/L
Application examples:
L-Fucose is present as the main component in fucoidan (a marine
polysaccharide), foods, pharmaceuticals and other materials
(e.g. biological samples, etc.)
Method recognition: Novel method
Advantages
Q1. Should the pH of the sample be adjusted even for samples in acidic media?
The pH of the assay solution after the sample is added should be the same as that of the assay buffer that is supplied with the kit.
Low sample volumes (e.g. 0.1 mL) are not likely to affect the pH of the assay solution and therefore may not require pH adjustment.
Samples above 0.1 mL are more likely to affect the pH of the assay solution and therefore the pH of these samples should be adjusted as described in the data booklet, prior to addition to the assay.
Q2. Sometimes a negative absorbance change is obtained for the blank samples, is this normal? Should the real value (negative absorbance change) or “0” be used in the calculation of results?
Sometimes the addition of the last assay component can cause a small negative absorbance change in the blank samples due to a dilution effect and in such cases it is recommended that the real absorbance values be used in the calculation of results.
Q3. There is an issue with the performance of the kit; the results are not as expected.
If you suspect that the Megazyme test kit is not performing as expected such that expected results are not obtained please do the following:
Q4. Can oligosaccharides or polysaccharides be measured using the kit assay?
The kit assay will only measure the non-covalently linked monosaccharide.
Oligosaccharides or polysaccharides can be measured after hydrolysis to the monosaccharide. Generally acid hydrolysis can be achieved by boiling the oligo/polysaccharide in 1.3 M HCl for 1 h. It is recommended that scientific literature is consulted for information on hydrolysis conditions for the particular oligo/polysaccharide that is being measured.
Q5. How can I work out how much sample to extract and what dilution of my sample should be used in the kit assay?
Where the amount of analyte in a liquid sample is unknown, it is recommended that a range of sample dilutions are prepared with the aim of obtaining an absorbance change in the assay that is within the linear range.
Where solid samples are analysed, the weight of sample per volume of water used for sample extraction/preparation can be altered to suit, as can the dilution of the extracted sample prior to the addition of the assay, as per liquid samples.
Q6. Can the sensitivity of the kit assay be increased?
For samples with low concentrations of analyte the sample volume used in the kit assay can be increased to increase sensitivity. When doing this the water volume is adjusted to retain the same final assay volume. This is critical for the manual assay format because the assay volume and sample volume are used in the calculation of results.
Q7. How much sample should be used for the clarification/extraction of my sample?
The volume/weight of sample and total volume of the extract can be modified to suit the sample. This will ultimately be dictated by the amount of analyte of interest in the sample and may require empirical determination. For low levels of analyte the sample:extract volume ratio can be increased (i.e. increase the sample and/or decrease the total extraction volume).
Alternatively, for samples with low concentrations of analyte, a larger sample volume can be added to the kit assay. When altering the sample volume adjust the distilled water volume added to the assay accordingly so that the total assay volume is not altered.
Q8. I have some doubts about the appearance/quality of a kit component what should be done?
If there are any concerns with any kit components, the first thing to do is to test the standard sample (control sample) that is supplied with the kit and ensure that the expected value (within the accepted variation) is obtained before testing any precious samples. This must be done using the procedure provided in the kit booklet without any modifications to the procedure. If there are still doubts about the results using the standard sample in the kit then send example results in the MegaCalc spread sheet to your product supplier (Megazyme or your local Megazyme distributor).
Q9. Can the test kit be used to measure biological fluids and what sample preparation method should be used?
The kit assay may work for biological fluids assuming that inositol is present above the limit of detection for the kit after any sample preparation (if required). Centrifugation of the samples and use of the supernatant directly in the kit assay (with appropriate dilution in distilled water) may be sufficient. However, if required a more stringent sample preparation method may be required and examples are provided at the following link:http://www.megazyme.com/docs/analytical-applications-downloads/biological_samples_111109.pdf?sfvrsn=2
The test kit has not been tested using biological fluids as samples because it is not marketed or registered as a medical device. This will therefore require your own validation.
Q10. Can the manual assay format be scaled down to a 96-well microplate format?
The majority of the Megazyme test kits are developed to work in cuvettes using the manual assay format, however the assay can be converted for use in a 96-well microplate format. To do this the assay volumes for the manual cuvette format are reduced by 10-fold. The calculation of results for the manual assay format uses a 1 cm path-length, however the path-length in the microplate is not 1 cm and therefore the MegaCalc spreadsheet or the calculation provided in the kit booklet for the manual format cannot be used for the micropalate format unless the microplate reader being used can.
There a 3 main methods for calculation of results using the microplate format:
Q11. When using this kit for quantitative analysis what level of accuracy and repeatability can be expected?
The test kit is extremely accurate – at Megazyme the quality control criteria for accuracy and repeatability is to be within 2% of the expected value using pure analytes.
However, the level of accuracy is obviously analyst and sample dependent.
Q12. Is it possible to add a larger volume then 2 μL of enzyme to the microplate assay? In some instances 2 μL can be difficult to pipette manually.
Yes, instead of adding 2 μL of enzyme suspension an alternative is to dilute the enzyme and add a larger volume to the microplate assay.
Dilute the assay buffer 10-fold with distilled water and use this as the diluent to dilute an aliquot of the enzyme suspension also by 10-fold. Instead of 2 μL, use 20 μL of the diluted enzyme in the microplate assay.
Q13. Must the minimum absorbance change for a sample always be at least 0.1?
No. The 0.1 change of absorbance is only a recommendation. The lowest acceptable change in absorbance can is dictated by the analyst and equipment (i.e. pipettes and spectrophotometer) and therefore can be can be determined by the user. With accurate pipetting, absorbance changes as low as 0.02 can be used accurately.
If a change in absorbance above 0.1 is required but cannot be achieved due to low concentrations of analyte in a sample, this can be overcome by using a larger sample volume in the assay to increase the absorbance change and thereby increase sensitivity of the assay. When doing this the increased volume of the sample should be subtracted from the distilled water volume that is added to the assay so that the total assay volume is unaltered. The increase sample volume should also be accounted for when calculating final results.
Q14. Can the sensitivity of the kit assay be increased?
Yes. Samples with the lower concentrations of analyte will generate a lower absorbance change. For samples with low concentrations of analyte, a larger sample volume can be used in the assay to increase the absorbance change and thereby increase sensitivity of the assay. When doing this the increased volume of the sample should be subtracted from the distilled water volume that is added to the assay so that the total assay volume is unaltered. The increase sample volume should also be accounted for when calculating final results.
