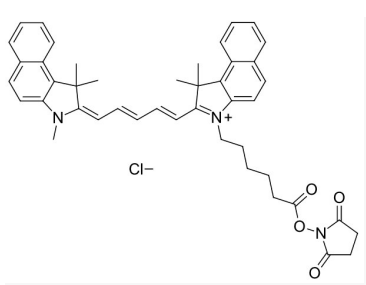
Cyhaiine5.5 NHS ester;Cy5.5 NHS ester;cas:2375105-86-3
Cy5.5-NHS ester;Cy5.5-琥珀酰亚胺/活化酯;Cyhaiine5.5-NHS ester;Cyhaiine5.5-琥珀酰亚胺/活化酯;

外观 :深蓝色固体
CAS号 :1469277-96-0
分子式 :C44H46ClN3O4
分子量 :716.31
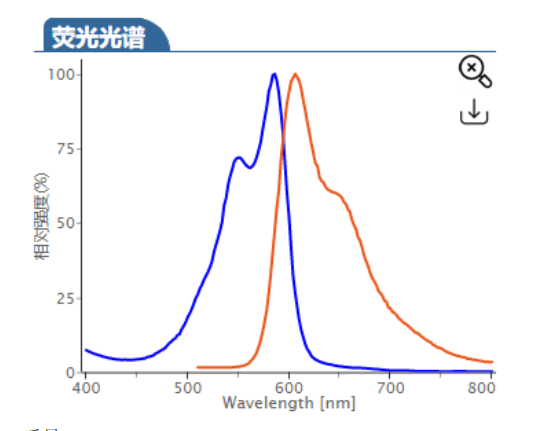
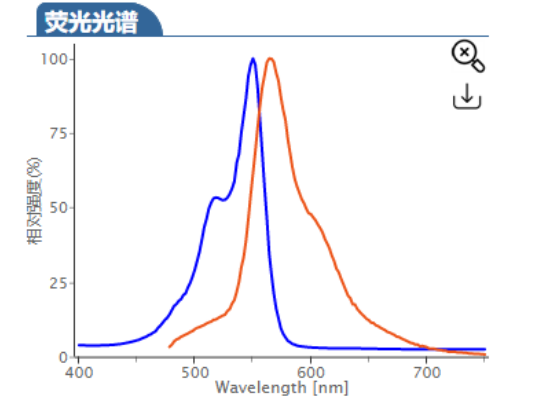
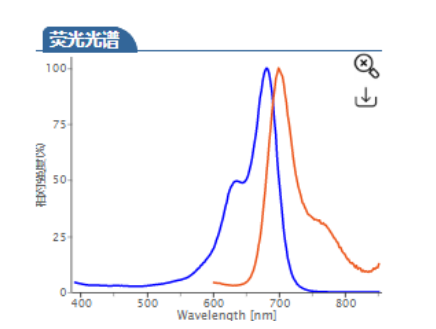
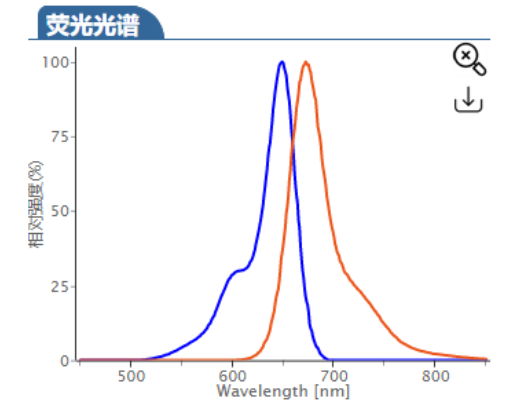
ex/em :680/698 nm (PBS缓冲液)
消光系数(ε) :209000 L⋅mol−1⋅cm−1
量子产率(Φ) :0.2
溶解性 :易溶于DMF等有机溶剂
保存条件 :避光,干燥,-20oC保存
运输条件 :室温
产品描述
Cy5.5 (Cyhaiine 5.5) 是一种发近红外(NIR)荧光的花青素荧光染料。它的消光系数高,荧光也很亮,并且对pH不敏感,并且由于它的荧光波长(Em:~700nm)恰好处于肌体组织近红外窗口 I 的区域(肌体的血液,体液和组织此区域背景荧光弱,而长波长穿透性强),所以Cy5.5常应用于小动物活体体内成像中。 Cy5.5可以用来标记蛋白,抗体,多肽,纳米粒等,它最常见的使用是标记核酸分子(DNA和RNA)。
NHS活化酯 (N-羟基琥珀酰亚胺酯,N-hydroxysuccinimide ester,succinimidyl ester)是生物标记反应中最常用的活化基团。它活化Cy5.5分子中的羧基,让它可以和目标生物分子上的胺基(伯胺或者仲胺)反应生成稳定的酰胺键,从而把Cy5.5分子标记到生物大分子上。由于游离胺基是蛋白,抗体,多肽表面的常见官能团(来自于赖胺酸侧链),Cy5.5-NHS可以和它们直接反应。核酸分子中天然不带游离胺基,只有胺基修饰过的RNA或者DNA才可以和Cy5.5-NHS反应。
Cy5.5-NHS活化酯的水溶性较低,所以在水相的标记反应体系内需要使用有机共溶剂,常用有机溶剂包括DMF,DMSO和乙腈等。如需标记的分子对有机溶剂敏感,可选择磺化花青素(sulfonated cyhaiine),即sulfo-Cy5.5-NHS活化酯。两者的光谱性质几乎一致,因此大部分应用可相互替换。两者的主要区别在于:磺化染料是水溶性的,在水相的标记反应体系内不需要使用有机共溶剂,并且降低染料在水中聚集的可能性,增强标记效率。
储存细则
Cy5.5-NHS活化酯固体可在-20°C保存一年,溶解后(无论水溶液还是无水有机溶剂)应尽快使用,不能长期保存。
质量: 99%+
包装: 10mg/50mg/100mg/500mg
保存条件: -20°C
保存时间: 2年
产地:西胺
用途:科研用

上海金畔生物科技有限公司主要提供Near IR Dyes 菁染料Cy3 Cy5 Cy7及其相关产品。
Sulfo-Cyhaiine 5 NHS ester
Sulfo-Cyhaiine3 azide
Sulfo-Cyhaiine3 carboxylic acid
Sulfo-Cyhaiine3 maleimide
Sulfo-Cyhaiine3 NHS ester
Sulfo-Cyhaiine5 alkyne
Sulfo-Cyhaiine5 azide
Sulfo-Cyhaiine5 carboxylic acid 1121756-16-8
Sulfo-Cyhaiine5 maleimide
Sulfo-Cyhaiine7 azide
Sulfo-Cyhaiine7 carboxylic acid
Sulfo-Cyhaiine7 NHS ester


 质量: 99%+
质量: 99%+