上海金畔生物科技有限公司是特级加拿大进口FBS品牌代理商 ,欢迎访问官网了解更多产品信息和订购。
特级加拿大进口FBS
详情介绍
特级加拿大进口FBS
产品名称:HyClone Characterized FBS, Canadian Origin, 500 mL, Irradiated 25-40kGy, Heat-inactivated;特级加拿大进口FBS
产品货号:SH30396.03IH25-40;
处理方式:3×100 nm滤膜过滤;
地区来源:加拿大;
物种来源:;
内毒素水平:内毒素水平:≤ 25 EU/mL;
血红素水平:< 25 mg/dL
热灭活:灭活;
射线处理:处理;
保存条件:≤-10℃;
运输方式:干冰运输;
详细介绍:A cost-efficient alternative to defined FBS, this high-quality Canadian serum is well-characterized, triple 100 nm filtered, and virus-tested to 9 CFR standard.
Characterized: assayed for a variety of critical components and biochemical characteristics.
Canadian origin: sourced in Canada and processed in the US with complete traceability.
Endotoxin-tested: certificate of analysis confirms ≤ 25 EU/mL endotoxin.
Tripled-filtered: serial 100 nm pore size-rated filterings for reliable microbial depletion.
Post-filtration treatments: γ-irradiated and heat-inactivated FBS options also available.
Virus-tested: broad virus panel testing according toin accordance with 9 CFR 113.53.
Fetal bovine serum (FBS) is a nutrient-rich cell culture supplement that is high in growth factors and low in antibodies, compared to non-fetal sera.
Characterized FBS for cell culture
HyCloneTM Characterized Fetal Bovine Serum (FBS) is an excellent alternative to more expensive defined sera, and meets the requirements of most discriminating FBS users. The final serum is assayed for provides important proteins, hormones, growth factors, metabolites, and nutrients for that support your healthy cell culture sprocess. Assay results are included in the certificate of analysis and biochemical assay list. Additional testing, including EMEA conformance testing, is also available.
Bacterial endotoxin-tested
Endotoxin contamination is a significant concern in research and manufacturing, especially when producing cell culture-derived products for use in humans or animals. The presence of endotoxin in cell cultures can also have adverse and unpredictable effects on cell growth, function, and phenotype that can compromise research results. Our patented closed-system blood collection method and rigorous quality control testing ensure that HyClone Characterized FBS meets industry standards of ≤ 25 EU/mL endotoxin. The final product also contains ≤ 25 mg/dL hemoglobin, which is an indicator of appropriate handling during collection and transport.
Virus-tested and quality-assured for reduced contamination risk
HyClone Characterized FBS is triple-filtered through serial 100 nm (0.10 μm) pore size-rated filters, which has become an industry standard for cell culture grade FBS. Before dispensing, each lot of serum is pooled using true pool technology to minimize variability from bottle to bottle.
The final serum is virus-tested according to 9 CFR 113.53 to demonstrate that there are no detectable levels of specified viruses, pathogenic agents, and hemadsorbing agents. Robust sterility testing further confirms that the final product contains no detectable growth of bacteria or fungi (current USP) and no detectable mycoplasma.
Post-filtration treatments for added process security
Gamma-irradiated and heat-inactivated FBS options are available to further reduce the risk of adventitious agents, particularly when manufacturing products for use in humans or animals. The irradiation process is validated to deliver a minimum dose of 25 kGy.
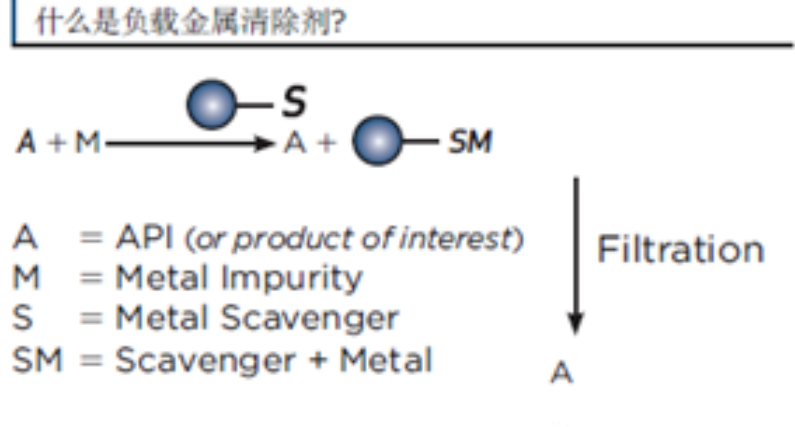
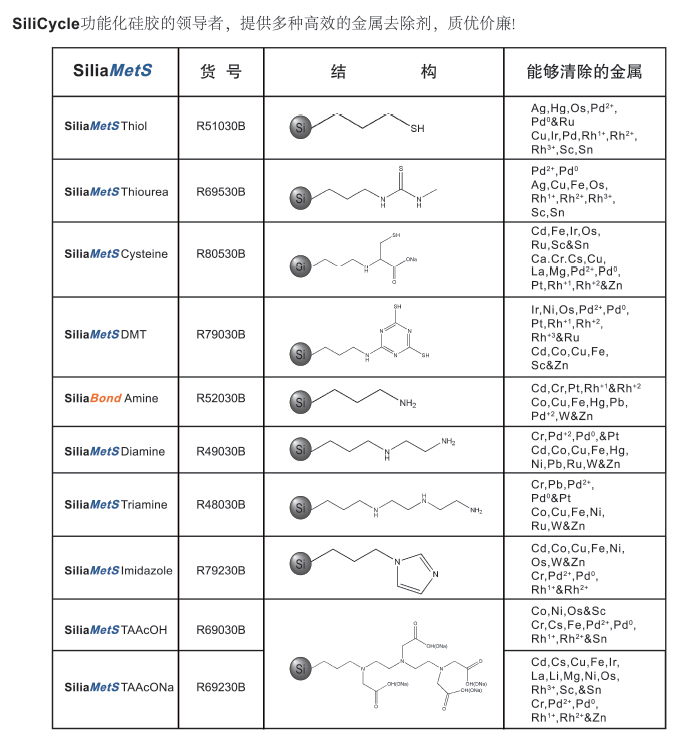
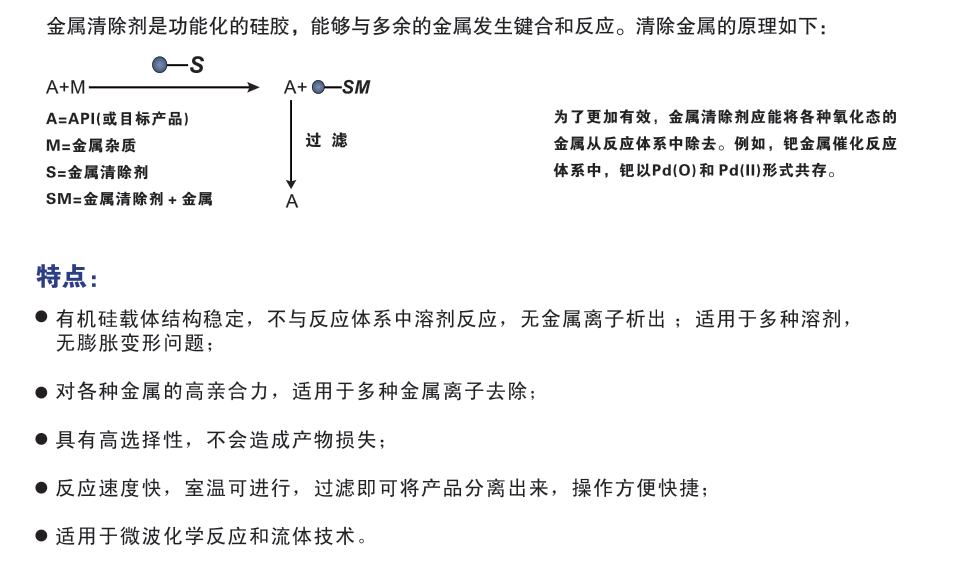 一、清除原理SiliaMetS
一、清除原理SiliaMetS