上海金畔生物科技有限公司提供各种分子量和基团修饰性聚乙二醇定制服务。
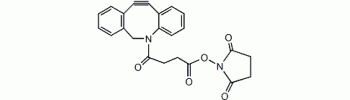
Dibenzylcyclooctyne, DBCO NHS
|
Description:
DBCO (Dibenzocyclooctyne) is a cycloalkyne that can reacts with azides via strain-promoted 1,3-dipolar cycloaddition in aqueous solution, a bioorthogonal reaction also termed Cu-free click reaction. This reaction has excellent selectivity and biocompatibility, such that the complimentary reagents can form covalent bonds with richly functionalized biological systems. The Cu-free click reaction has been a powerful tool in catalyst-free bioconjugation. DBCO reagents posses fast kinetcs and stability in aqueous buffer and they can be used to label azide-modified biomolecules with high specificity and reactivity.
Physical Properties:
- Yellow/pale-yellow solid or viscous liquid;
- Soluble in DMSO or DMF;
Storage Conditions:
- Store at -20 0C, desiccate. NHS tends to hydrolyze from moisture. Avoid frequent thaw and frozen.
Reaction Procedures:
NHS esters are moisture senstivie. To avoid moisture condensation onto the product always let vial come to room temperature before opening; be exposure to limit exposure to moisture and restore under atmosphere. The NHS ester moiety readily hydrolyzes and becomes non-reactive; therefore, prepare stock solutions immediately before use. Stock solutions in anhydrous solvents can be kept for several days (freeze when not in use). Hydrolysis of the NHS ester is a competing reaction. Conjugation with primary amines of proteins/peptides (i.e., acylation) is favored at near neutral pH (6-9) and with concentrated protein solutions. For conjugation, use non-amine containing buffers at pH 7-9 such as PBS (20 mM sodium phosphate, 150 mM sodium chloride, pH 7.4); 20 mM HEPES; 100 mM carbonate/biocarbonate; or 50 mM borate buffer. Do not use buffers that contain primary amines, (e.g., Tris, glycine). Avoid buffers that contain azides, which can react with DBCO. Dissolve DBCO water miscible organic solvent such as DMSO or DMF before diluting in final reaction buffer. DBCO-NHS ester is not soluble in aqueous buffers.
Materials Required:
- Conjugation buffer: Sodium bicarbonate 100 mM buffer, pH 8.5 or other amine-free buffer at pH 7-8.5.
- Water miscible solvents: DMSO or DMF.
- Quenching buffer: 1 M Tris.HCl, pH 8.0.
Reaction Steps:
Prepare proteins in PBS. Immediately before use, prepare 10 mM of the DBCO-NHS reagent in DMSO or DMF. Add the NHS reagent to the protein sample at a final: mM. If For samples <5 mg/ml, use a 20 to 50 fold molar excess. Incubate the reaction at room temperature for 30 minutes or on ice for 2 hours. Stop the reaction by adding Quenching Buffer 100 mM Tris.or on ice for 15 minutes. Remove non reagentby dialysis or desalting.
Copper-free Click Reaction:
Prepare the azide-containing sample in reaction buffer. Add DBCO conjugate to azide containing sample. Recommendation: Add 1 mole equivalent of limiting reagent to 1.5-3.0 mole equivalents of highest abundance reagent. Incubate the reaction at room temperature for 2-4 hours or 2-12 hours at 4 degree celcius. The reaction is now ready for purification.